Table of Contents
ToggleIntroduction
The Stem Cell Manufacturing Market encompasses the technologies, processes, and services involved in producing stem cells and stem cell–derived products at clinical and commercial scales. Stem cell manufacturing supports regenerative medicine, gene and cell therapies, tissue engineering, and personalized medicine by enabling consistent, high-quality production of pluripotent, multipotent, and engineered cell populations. As the global healthcare landscape shifts toward curative treatments for chronic and degenerative diseases, scalable, reproducible, and compliant stem cell manufacturing has become essential for therapeutic success.
Market Size and Growth Projections
The stem cell manufacturing market is experiencing rapid expansion, driven by increasing investments in cell therapy R&D, regulatory approvals of advanced cellular therapies, and rising demand for standardized and scalable production solutions. The market is projected to grow at a robust compound annual growth rate (CAGR) over the forecast period as stakeholders prioritize Good Manufacturing Practice (GMP) capabilities, automation, and integrated bioprocessing platforms. Heightened collaboration between biotech firms, contract manufacturing organizations (CMOs), and academic institutions further propels market valuation.
Get More Details : https://www.databridgemarketresearch.com/reports/global-stem-cell-manufacturing-market
Key Growth Factors
-
Rising development and commercialization of cell and gene therapies
-
Increasing prevalence of chronic and degenerative diseases
-
Growth in GMP-compliant manufacturing infrastructure
-
Demand for scalable, automated, and reproducible production
-
Strategic investments and partnerships between industry and research institutions
Market Segmentation
By Type / Service / Product
The market is segmented into:
-
Raw Materials and Reagents (culture media, growth factors, cytokines)
-
Cell Processing and Expansion Systems (bioreactors, cell culture platforms)
-
Separation and Purification Technologies
-
Cryopreservation and Storage Solutions
-
GMP Facility Services and Contract Manufacturing Services
Cell processing and expansion systems are critical due to their direct impact on cell yield, viability, and quality, while GMP facility services support outsourced production and compliance.
By Application
Based on application, the stem cell manufacturing market includes:
-
Regenerative Medicine & Therapeutics
-
Cancer Immunotherapy and Hematological Disorders
-
Tissue Engineering and Organ Repair
-
Drug Discovery & Toxicology Testing
-
Cosmetic and Dermatological Applications
Regenerative medicine and therapeutic development represent major growth areas, driven by clinical demand for curative treatments.
By End User
End users consist of:
-
Biotechnology and Pharmaceutical Companies
-
Contract Development & Manufacturing Organizations (CDMOs/CMOs)
-
Academic and Research Institutions
-
Hospitals and Clinical Centers
-
Cell Therapy Developers
Biotech and pharmaceutical companies, along with CDMOs/CMOs, lead market adoption due to resource needs for scale-up, regulatory compliance, and clinical production.
Regional Insights
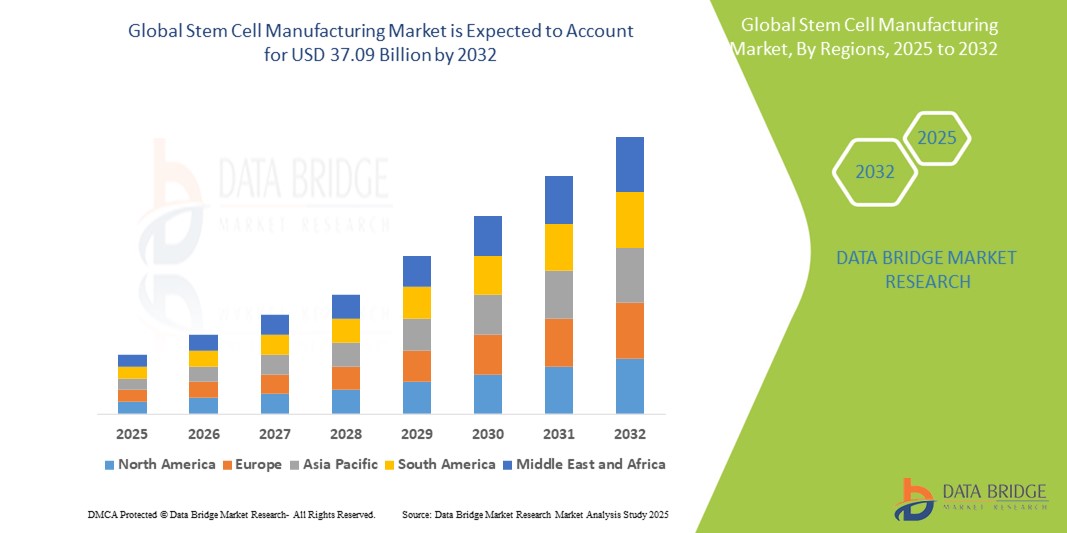
North America dominates the stem cell manufacturing market with advanced R&D infrastructure, supportive regulatory frameworks, and significant investment in cell therapy pipelines. Europe follows with strong academic research networks, public-private partnerships, and emerging GMP facilities. The Asia-Pacific region is rapidly expanding as countries like China, Japan, and South Korea invest in regenerative medicine, bioprocessing infrastructure, and streamlined regulatory pathways. Latin America and Middle East & Africa demonstrate nascent growth with increasing collaborations and capacity building.
Key Market Drivers
The stem cell manufacturing market is driven by the increasing clinical adoption of cell-based therapies for conditions such as neurodegenerative diseases, diabetes, cardiovascular disorders, and orthopedic injuries. Regulatory emphasis on product quality, safety, and reproducibility necessitates advanced manufacturing platforms that ensure consistent performance. Additionally, innovations in automation, closed processing systems, and digital bioprocess monitoring reduce contamination risk, lower labor costs, and support scalable production.
Market Challenges and Restraints
Despite promising prospects, the market faces challenges including high capital expenditures for GMP facilities and advanced bioprocess equipment, complex regulatory landscapes across regions, and skills shortages in cell manufacturing expertise. Batch-to-batch variability, supply chain constraints for critical reagents, and intellectual property complexities can further impede rapid scale-up. Ensuring long-term cell stability and function during storage and transport remains a technical hurdle.
Competitive Landscape with Key Companies
-
Thermo Fisher Scientific Inc.
-
Merck KGaA (MilliporeSigma)
-
GE Healthcare (Cytiva)
-
Lonza Group AG
-
Sartorius AG
-
Fujifilm Holdings Corporation (Cellular Dynamics)
-
Miltenyi Biotec
-
REPROCELL
These companies emphasize GMP-compliant solutions, integrated bioprocess platforms, strategic partnerships, and contract manufacturing services to support innovators in advanced therapies and regenerative applications.
Technological Innovations
Technological advancements transforming the stem cell manufacturing market include use of single-use bioreactors, automated closed-loop processing systems, real-time process analytics (PAT), and AI-enabled bioprocess optimization. Innovations in microcarrier technologies, 3D culture systems, and scalable expansion platforms improve cell yields and quality. Progress in cryopreservation formulations and controlled rate freezers enhances storage stability and logistics efficiency.
SWOT Analysis
| Strengths | Weaknesses |
|---|---|
| Supports next-gen therapies and regenerative medicine | High cost of GMP infrastructure and technologies |
| Enables scalable, reproducible production | Complex regulatory compliance requirements |
| Strong cross-industry collaborations | Skills gap in advanced cell manufacturing |
| Opportunities | Threats |
|---|---|
| Expansion of CDMO/CMO services | Regulatory unpredictability across regions |
| Growth in personalized medicine and autologous therapies | Supply chain vulnerabilities for reagents |
| Integration of automation and AI | Competitive pressures from alternative platforms |
Future Market Outlook
The future outlook for the stem cell manufacturing market is strongly positive as cell and gene therapies progress through clinical pipelines and gain regulatory approval. Demand for standardized, reproducible, and cost-effective manufacturing will drive adoption of modular, automated platforms that reduce overhead and accelerate time to market. Expansion of global CDMO networks, digital bioprocessing solutions, and collaborative innovation ecosystems will enable broader therapy accessibility and commercial scalability.
Conclusion
The Stem Cell Manufacturing Market is poised for sustained growth and strategic importance as healthcare systems embrace regenerative, curative, and personalized therapies. With increased investment, technological innovation, and regulatory alignment, stem cell manufacturing solutions are becoming foundational to next-generation biotechnology and therapeutic modalities. Continued focus on scalability, quality assurance, and cost-efficient production will shape the trajectory of this high-impact marketplace.